
The transformation of Grade 5 Titanium into a material as strong as diamond.
How do you take military-grade titanium,
a metal with the best strength to weight ratio and make it even stronger?
You create something entirely new.
What is Tyamine®
Tyamine® is a proprietary amorphous carbon coating made up of a unique combination of graphite and diamond crystal structures. It offers unmatched scratch and corrosion protection with zero compromises.
How is this possible?
Tyamine® forms a precise layering of microscopic diamond cubic structures held together with honeycomb graphite structures. The diamond structures act as the strong bricks offering the ultimate protection, while the graphite acts as the mortar locking them in place.
Carbon is the principal component of both diamonds and graphite, but their looks and properties are quite different. Diamonds are cubic arrangements of carbon that form rigid crystal structures. The diamond ranks a 10 on the Mohs hardness scale, making it one of the hardest materials on earth. It is a brilliant gem that acts as a prism of light of unsurpassed strength.
By comparison, graphite is composed of a flat layer of carbon in a honeycomb pattern and is extremely strong when pulled laterally but is easily cleaved if stacked on top of one another. Unlike diamond, it is softer and slicker, ranking a 1 on the Moh’s scale, making it suitable for use in pencils when stacked vertically. Additionally, graphite’s structure permits electrons to move freely within the particle, making it a very efficient conductor of heat, electricity, and light, which is why it appears black, unlike a diamond. However, it is the lateral strength of graphite that is utilised to bind together the rigid diamond structures.
The Difference


It is how the carbon atoms are arranged; though both materials are comprised of pure carbon, they are arranged in different structures, making them carbon allotropes (different structural forms of the same element).
In a diamond, the carbon atoms are arranged tetrahedrally. Each carbon atom is attached to four other carbon atoms 1.544 x 10-10 meters away with a C-C-C bond angle of 109.5 degrees. This creates a strong three-dimensional network of atoms. This rigid network of atoms accounts for a diamond’s extraordinary durability, hardness, and strength.

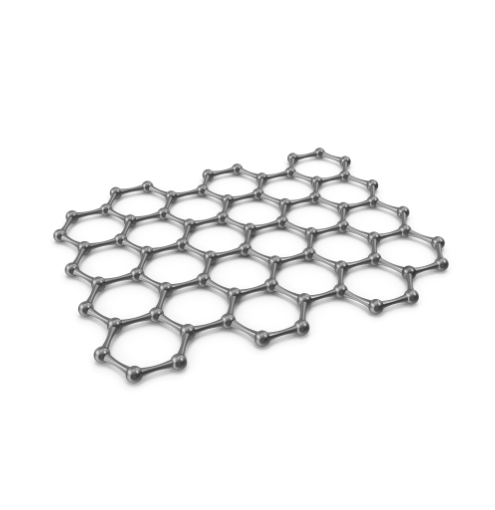
Graphene also comprises an infinite array of connected carbon atoms, but unlike diamonds, they are layered. Each carbon atom has two types of interactions with one another. First, each carbon atom is bonded with three other carbon atoms and arranged in the corners of a regular hexagon with a 120-degree C-C-C bond angle. This array extends in two dimensions to form a horizontal, hexagonal ‘honeycomb’ array. The three-dimensional structure of graphene is created when the two-dimensional arrays of carbon atoms are held together by the second weaker force of stacking interaction.
The unique combination of these two carbon structures and other elements has created Tyamine®, an intricate cobblestone of carbon structures as strong as a diamond with the pliability and lightness of graphite.
How is the Tyamine® coating applied?
Tyamine® can be applied to any conductive metal, but it is more effective used in combination with titanium, the lightest yet strongest metal available. Once a piece of titanium is ready to be coated, it goes through the following process.
Preparation
The piece is moved to a stainless steel vacuum
chamber and placed on a carousel. The chamber is
then heated to 150°C (300°F) to ensure no moisture
remains within the chamber or on the material
during the coating process.
The material then undergoes an ion bombardment
to remove any micro oxides or impurities on the
surface of the metal. For the best possible finish,
every feasible step must be taken to prime the
material’s surface.
Coating
To begin the coating process, hydrogen gas carrying
our proprietary carbon formula is introduced into the
chamber. This gas is then ionised by auxiliary high-
voltage electrical anodes, causing the carbon and
hydrogen atoms to split.
Carbon atoms are attracted to the material when an
opposing electrical current flows through the
carousel as it begins to spin. This electrical charge
pulls the carbon onto the material creating a
cobblestone of diamond and graphite carbon
structures.
The rotation of the carousel is crucial to ensuring that
every facet of the piece is evenly coated with the
Tyamine® finish.
Tyamine® provides unparalleled scratch and
corrosion resistance while retaining the weight and
impact resistance of titanium. Pushing Military-grade
titanium to new heights of strength, resilience and
utility.
Currently the application of Tyamine® is available by invitation only.
If you are interested in working with Tyamine®, please complete the contact form.
Thanks for submitting your information!
We will be in touch with you shortly.
